Written by Jeffrey Gardner, associate professor of biological sciences, UMBC
One of the scariest things you can be told when at a doctor’s office is “You have an antimicrobial-resistant infection.” That means the bacteria or fungus making you sick can’t be easily killed with common antibiotics or antifungals, making treatment more challenging. You might have to take a combination of drugs for weeks to overcome the infection, which could result in more severe side effects.
Unfortunately, this diagnosis is becoming more common around the world.
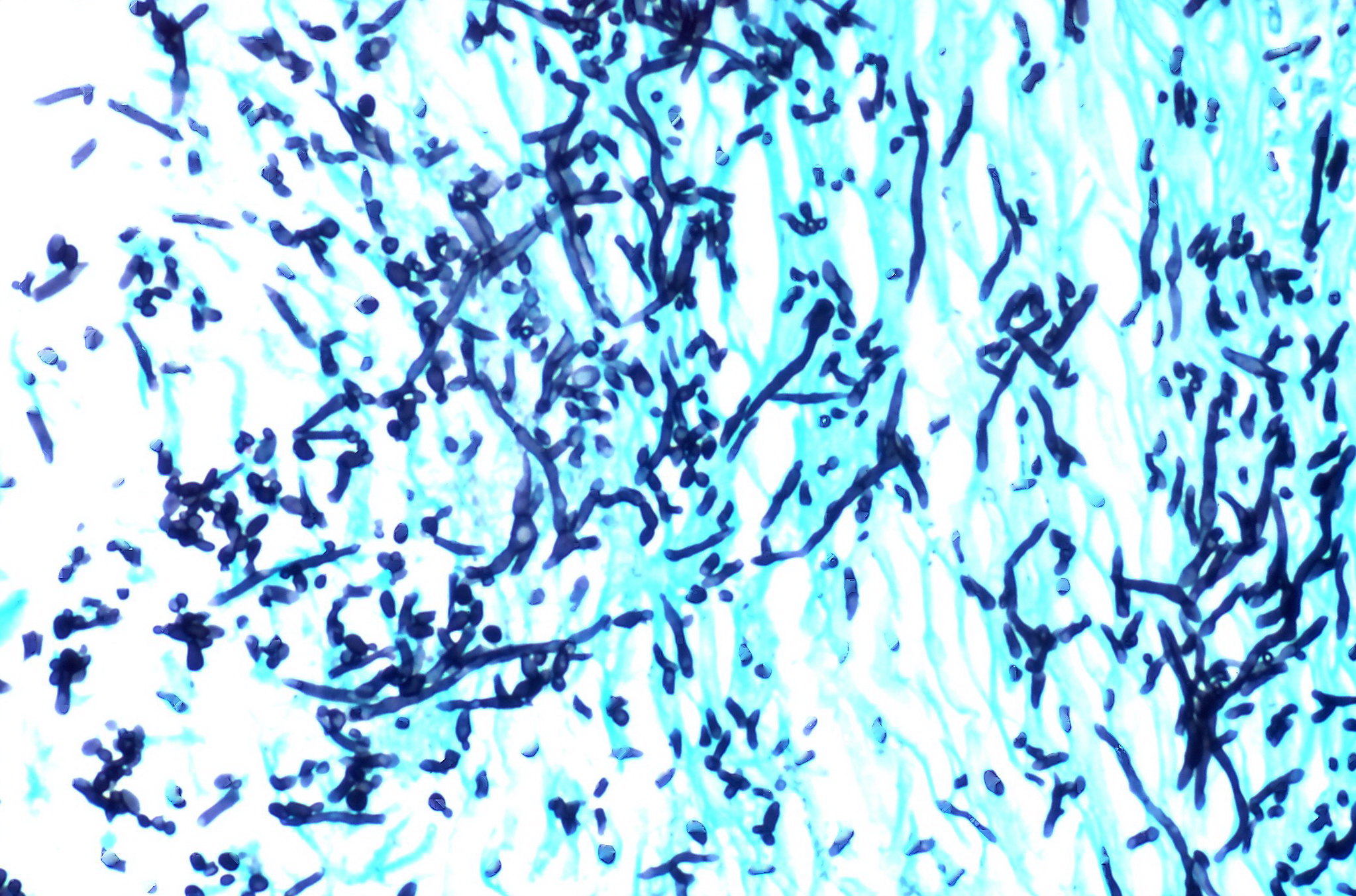
The yeast Candida auris has recently emerged as a potentially dangerous fungal infection for hospital patients and nursing home residents. First discovered in the late 2000s, Candida auris has very quickly become a major health challenge due to its ease of spread and ability to resist common antifungal drugs.
How did this fungus become so strong, and what can researchers and physicians do to combat it?
I am a microbiologist researching new ways to kill fungi. Candida auris and other fungi use three common cellular tricks to overcome treatments. Luckily, exciting new research hints at ways we can still fight this fungus.
Targeting the sensitive parts of fungal cells
Fungal cells contain a structure called a cell wall that helps maintain their shape and protects them from the environment. Fungal cell walls are constructed in part from several different types of polysaccharides, which are long strings of sugar molecules linked together.
Two polysaccharides found in almost all fungal cell walls are chitin and beta-glucan. The fungal cell wall is an attractive target for drugs because human cells do not have a cell wall, so drugs that block chitin and beta-glucan production will have fewer side effects.
Some of the most common drugs used to treat fungal infections are called echinocandins. These drugs stop fungal cells from making beta-glucan, which significantly weakens their cell wall. This means the fungal cell can’t maintain its shape well. While the fungus is struggling to grow or is breaking apart, your immune system has a much better chance of fighting off the infection.
How fungi become drug resistant
Unfortunately, some strains of Candida auris are resistant to echinocandin treatment. But how does the fungus actually do it? For decades, scientists have been studying how fungi overcome drugs designed to weaken or kill them. In the case of echinocandins, Candida auris commonly uses three tricks to beat these treatments: hide, build and change.
The first trick is to hide in a complex mixture of sugars, proteins, DNA and cells called a biofilm. Made with irregular 3D structures, biofilms have lots of places for cells to hide. Drugs aren’t good at penetrating biofilms, so they can’t access and kill cells deep inside. Biofilms are especially problematic when they grow on medical equipment like ventilators or catheters. Once free of a biofilm, cells that have gained the ability to resist the drugs a patient was taking become more dangerous.
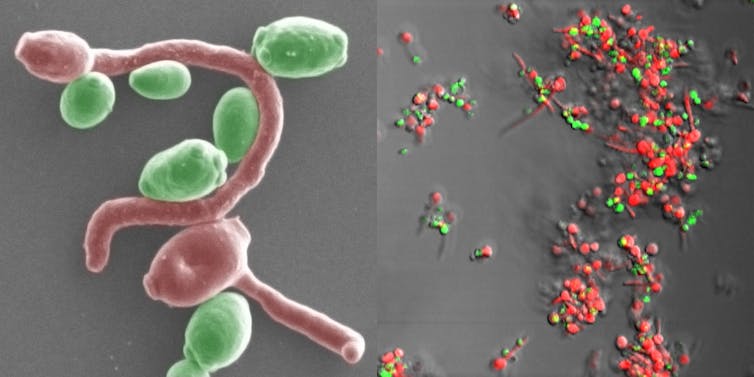
The second trick fungi use to evade treatment is to build cell walls differently. Fungal cells treated with echinocandins can’t make beta-glucan. So instead, they start to make more chitin, another important polysaccharide in the fungal cell wall. Echinocandins are unable to stop chitin production, so the fungus is still able to build a strong cell wall and avoid being killed. While there are some drugs that can stop chitin production, none are currently approved for use in people.
The third trick fungi rely on is to change the shape of the beta-glucan production enzyme so echinocandins cannot block it. These mutations allow beta-glucan production to continue even in the presence of the drug. It is not surprising that Candida uses this trick to resist antifungal drugs since it is very effective at keeping the cells alive.
New tactics to fight fungi
What can be done to treat echinocandin-resistant fungal infections? Thankfully, scientists and physicians are researching new ways to kill Candida auris and similar fungi.
The first approach is to find new drugs. For example, there are two drugs in development, rezafungin and ibrexafungerp, that appear to be able to stop beta-glucan production even in fungi resistant to echinocandins.
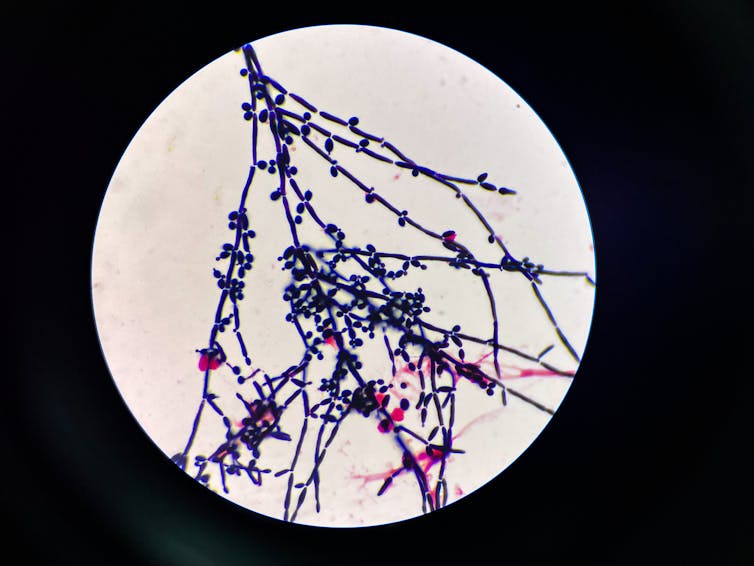
A complementary approach my research group is exploring is whether a class of enzymes called glycoside hydrolases might also be able to combat drug-resistant fungi. Some of these enzymes actively destroy the fungal cell wall, breaking apart both beta-glucan and chitin at the same time, which could potentially help prevent fungi from surviving on medical equipment or on hospital surfaces.
My lab’s work on discovering enzymes that strongly degrade fungal cell walls is part of a new strategy to combat antifungal resistance that uses a combination of approaches to kill fungi. But the end goal of this research is the same: having a physician tell you, “You’ve got a fungal infection, but we have a good treatment for it now.”
This article is republished from The Conversation under a Creative Commons license. Read the original article and see more than 250 UMBC articles available in The Conversation.
Tags: Biology, CNMS, Research, The Conversation