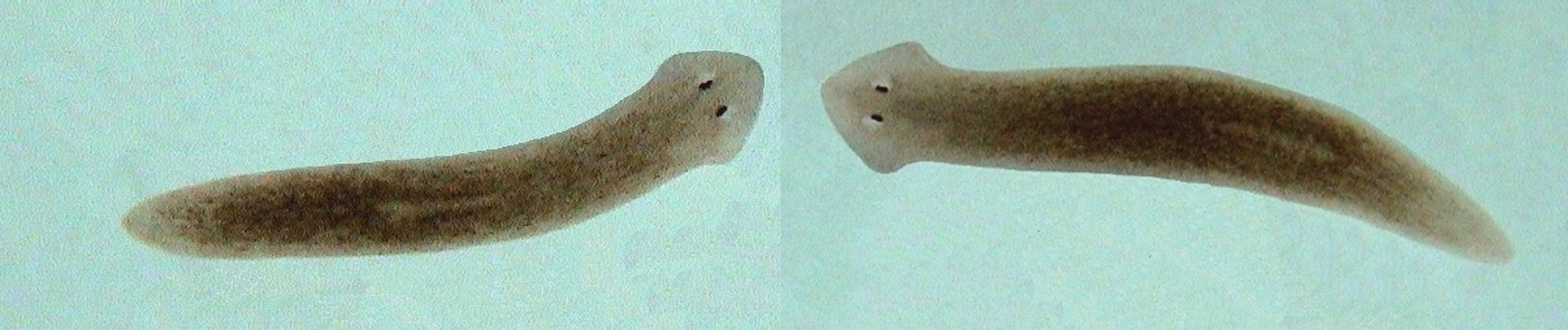
Other scientists thought it couldn’t be done. One called it “ridiculously impossible.” But Michael Levin, a biology professor at Tufts University, disagreed. Levin hired Daniel Lobo, now an assistant professor of biology at UMBC, to help solve a biological mystery: How does a flatworm grow a head here and a tail there? How can it regenerate a complete body from less than one percent of its original form? What could explain the tiny creature’s amazing feats of regeneration?
Levin saw that Lobo had “the right combination of computer expertise and interest in biology” to tackle a challenge like this, writes Cynthia Graber in “Replicating Life in Code” on PBS.org. So Levin took Lobo on as a postdoc and he got to work.
First, Lobo had to turn all the existing scientific data about flatworm growth (more than 1,000 experiments) into a database that a computer could understand. “Lobo created a standardized language and a standardized mathematical approach that represents the shapes of the worm’s regions, its organs, and how they’re interconnected,” writes Graber. “The end result was a searchable database of results, which is now available to all flatworm biologists.”
Next, he wrote the simulation program, “a virtual worm on which candidate models would test their results,” writes Graber. To run the program, Lobo and Levin rented time on a supercomputer owned by the University of Texas. As the program runs, “the models receive scores based on how well they predict the outcomes seen in flesh-and-blood flatworms,” Graber explains. If a model more closely matches reality, the program allows it to “reproduce,” or recombine its characteristics with other models, just like in real-life evolution. Then the next generation of models is tested against the database. The process continues until the program generates a model that matches all the data.
After six billion simulated experiments and 26,727 generations, a model emerged that matched every real-life experiment. It predicted experiments that had never been done, so Lobo and team tested them and found the model had predicted the results accurately.
Lobo’s success led him to explore other applications for this type of modeling approach. A graduate student in Levin’s lab ran experiments to collect data about pigmentation in tadpoles (as a model for melanoma), and then Lobo created a program that evolved to model why some tadpoles develop cancer and others don’t. The winning model predicted the results of all but one existing scientific paper. The team reran that experiment and got a different result—one that agreed with the model.
In his UMBC lab, Lobo uses similar modeling methods to discover how bacteria produce certain compounds. Bacteria already produce some therapeutic compounds, such as insulin. If scientists could trigger bacteria to produce other compounds based on what we learn from computer models, the results could revolutionize drug development. Lobo is also now “trying to reverse-engineer cancer tumors to attempt to discover the best possible treatments to cause them to collapse,” writes Graber.
Lobo and Levin agree that using artificial intelligence in the form of evolving computer models to answer biological questions is a major field of growth with tremendous potential. Lobo is on the cutting edge of this work, where computer science and biology meet.
Read more in “Replicating Life in Code” by Cynthia Graber on pbs.org.
Image: Planaria, a variety of flatworm commonly used as a model organism in experiments; photo by Jennifer Connelly.